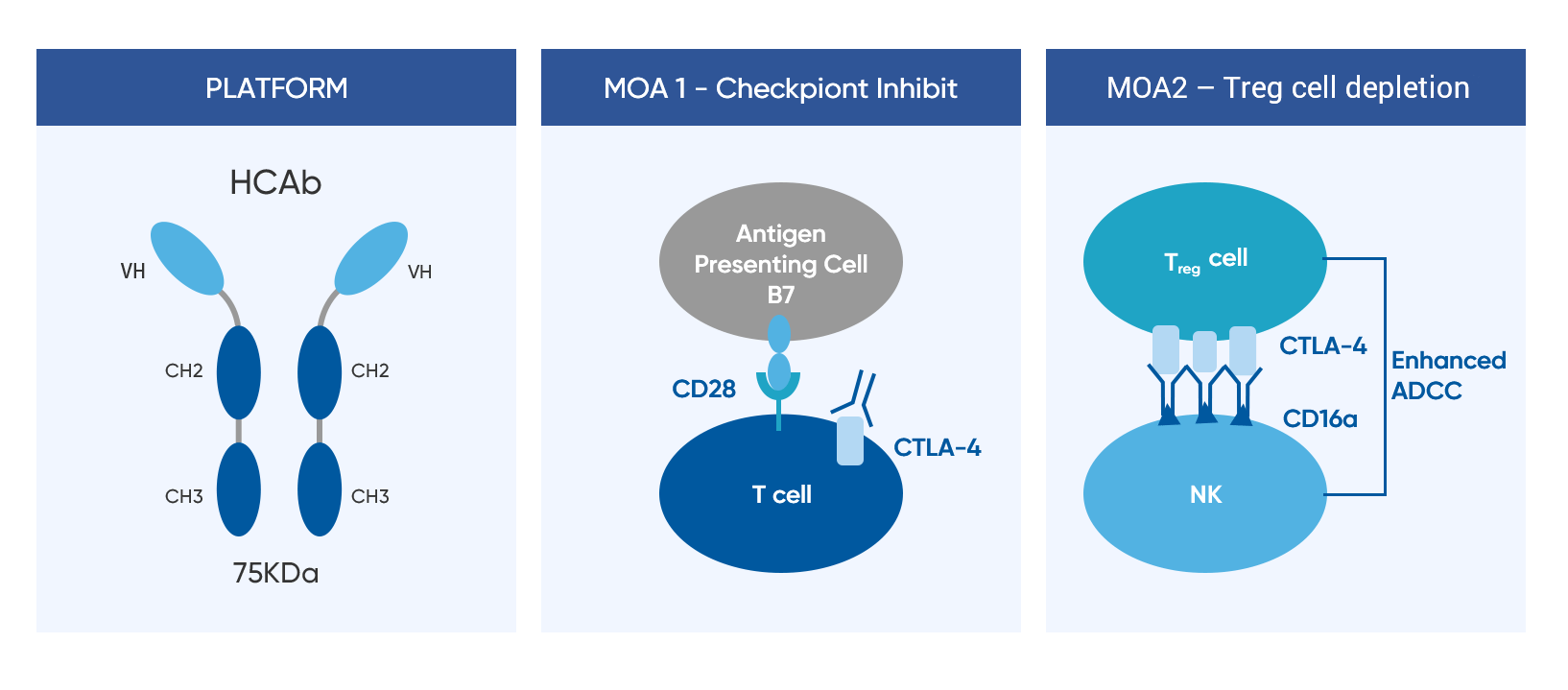
Porustobart (HBM4003) is a fully human anti-CTLA-4 monoclonal heavy chain only antibody (HCAb) generated from Harbour Mice®. By enhancing antibody-dependent cell cytotoxicity (ADCC) killing activity, porustobart has demonstrated significantly improved depletion specific to high CTLA-4 Treg cells in tumor tissues. The potent anti-tumor efficacy and differentiated pharmacokinetics with durable pharmacodynamic effect presents a favorable product profile. This novel and differentiated mechanism of action has the potential to improve efficacy while significantly reducing the toxicity of the drug in monotherapy and combo-therapy.
As the first heavy chain only fully human antibody on clinical stage in the world, porustobart showed its good safety profile and strong efficacy on its monotherapy study of phase I trial. HBM is making all efforts to push forward to the studies of this product as treatment for multiple solid tumors, including melanoma, NSCLC, HCC, NET/NEC.
Mechanism of Action
Porustobart stimulates the immune system by 2 mechanisms: inhibition of negative signaling from the interaction of CTLA-4 with the co-stimulatory molecule B7; and depletion of immune suppressive Treg in tumor tissues through enhanced ADCC.