Harbour BioMed has established a robust pipeline in oncology and immunology since the company was established in 2016. The current portfolio includes five clinical-stage in-licensed compounds and a rapidly emerging set of therapeutics coming from its internal discovery efforts as well as co-discovery/development collaborations with academic institutions and biopharmaceutical companies.
contact usAbout HBICE®
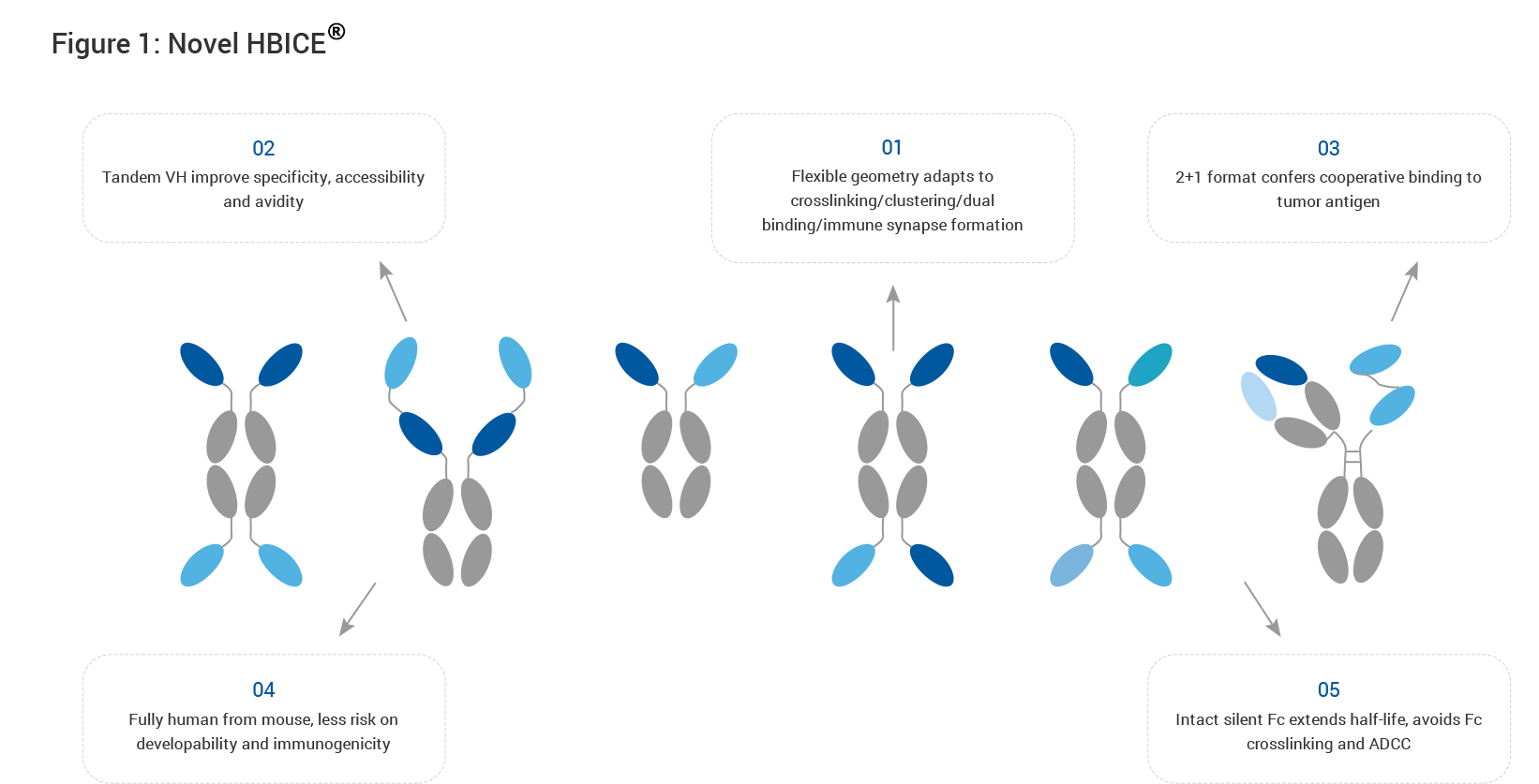
Harbour HCAb platform can generate diverse and stable fully human Heavy Chain only Antibodies (HCAbs) and derived human VH single-domain moieties, enabling us to make novel multi-specific and multi-valent antibodies in simplified structures with relatively smaller molecule size and fewer number of polypeptide chains. On top of this, we have established proprietary HBICE® (HCAb Based Immune Cell Engagers) platform to quickly develop multi-specific antibodies that redirect immune cells to the tumor microenvironment (TME) to eradicate tumors.
HBICE® molecules recognize and bind both specific tumor associated antigens (TAA) on tumor cells and CD3 or co-stimulatory molecules on immune cells such as T cells or NK cells, resulting in efficient and selective activation of immune cells in the TME, thereby preventing non-specific activation of peripheral immune cells. Besides, HBICE® technology provides the flexibility to generate molecules with different architectures and avidity to achieve different mechanisms of action that are unachievable by combo therapies.
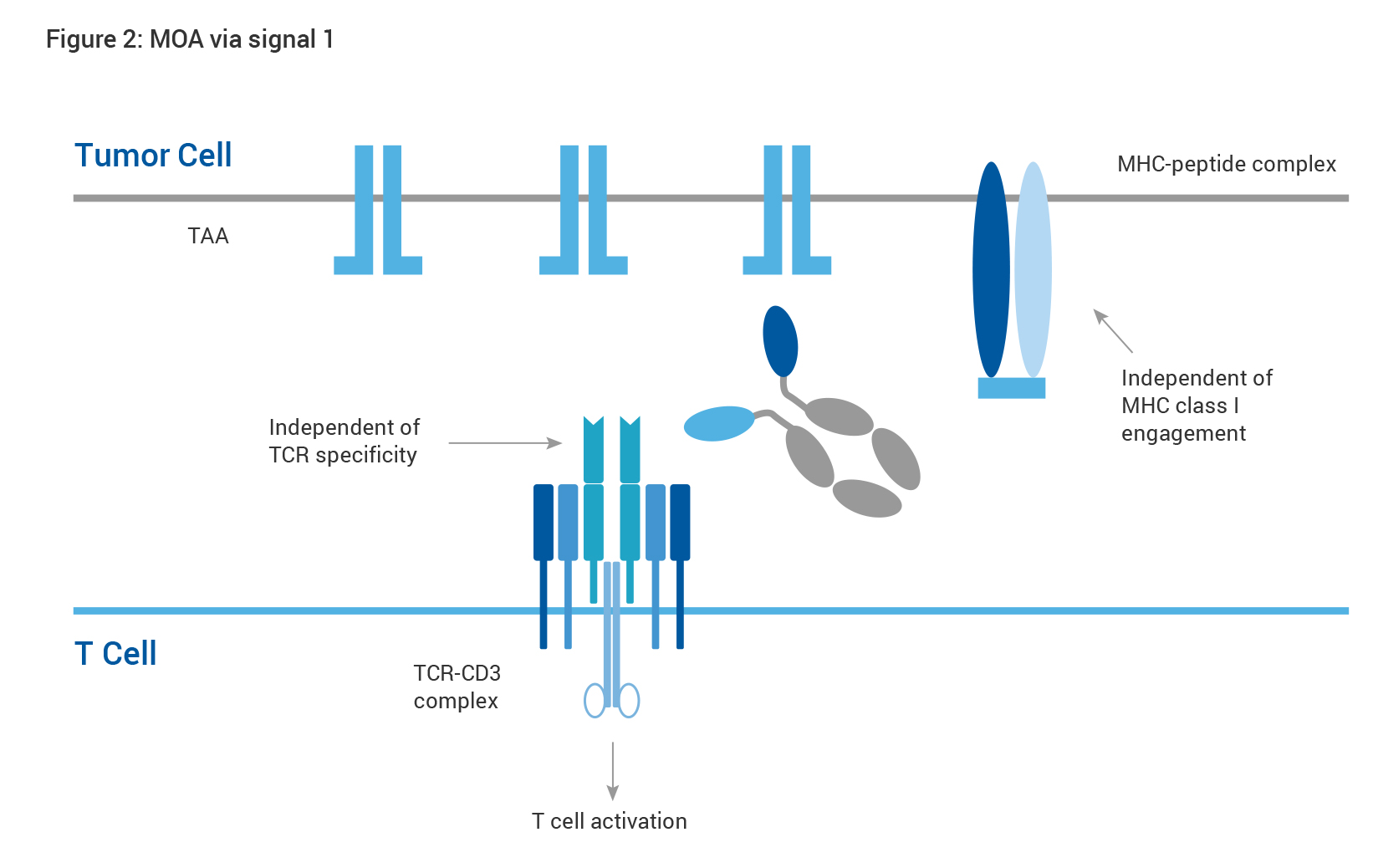
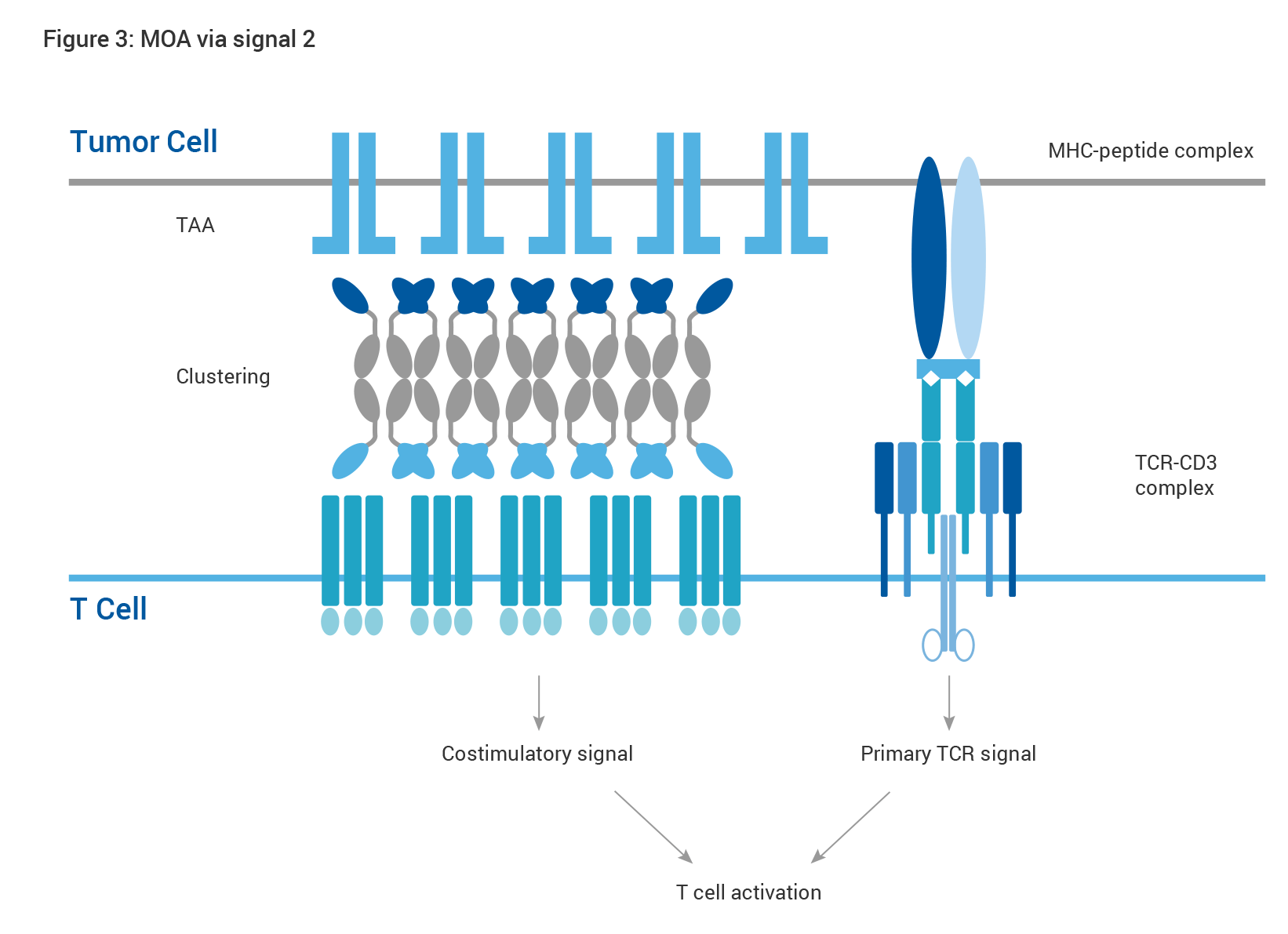
Exemplary scenarios are depicted by the diagrams below (Figs. 2 and 3). Bypassing the conventional T-cell activation pathway trigged by TCR-MHC interaction, CD3-targeting HBICE® molecules can elicit a polyclonal T-cell response to overcome immune escape mechanisms such as downregulation of antigen presentation in a non-MHC restricted fashion (Fig. 2). T cells in the TME are often in sub-optimal condition in absence of the co-stimulatory signal. Supplementation of HBICE® antibodies targeting the co-stimulatory molecules results in TAA-mediated clustering of co-stimulatory molecules and subsequent activation of the downstream pathway, providing a costimulatory signal for full activation of T cells, which leads to effective tumor eradication as well as improved safety profile (Fig. 3).