The antibody formats produced by the HCAb mice have shown desirable expression yields and biophysical properties, such as solubility, non-aggregation and thermal stability, allowing for use either as simplified alternatives to conventional antibodies or as components of more complex antibody products.
Harbour Mice® – Generating Human Antibodies for Therapy
Transgenic mice have emerged as powerful tools for use in biologic drug discovery based on their unique potential in producing fully human antibodies. The majority of fully human antibodies approved to date in the U.S. have emerged from one or the other transgenic mice platform.
Harbour BioMed's biologic discovery programs are based on two proprietary transgenic mouse platforms for generating human therapeutic antibodies. The platforms have broad potential for generating both conventional as well as the Next-Gen biologics that are fully human, affinity matured with excellent solubility and developability.
The transgenic mouse platforms are:
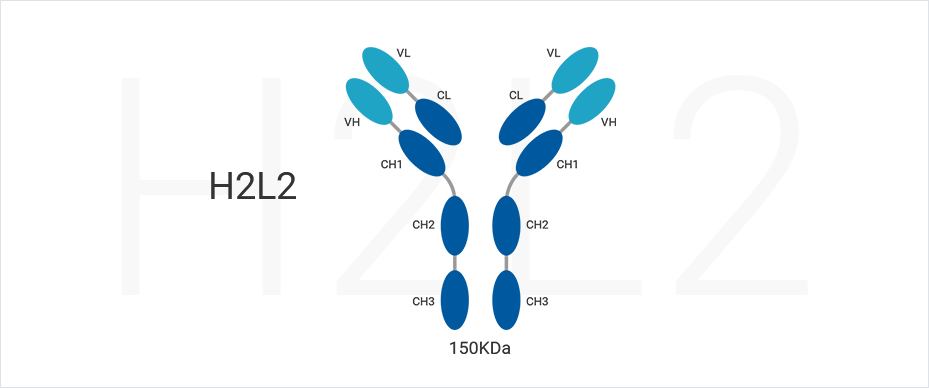
H2L2 mice generates traditional tetrameric antibodies comprised of two heavy chains and two light chains.
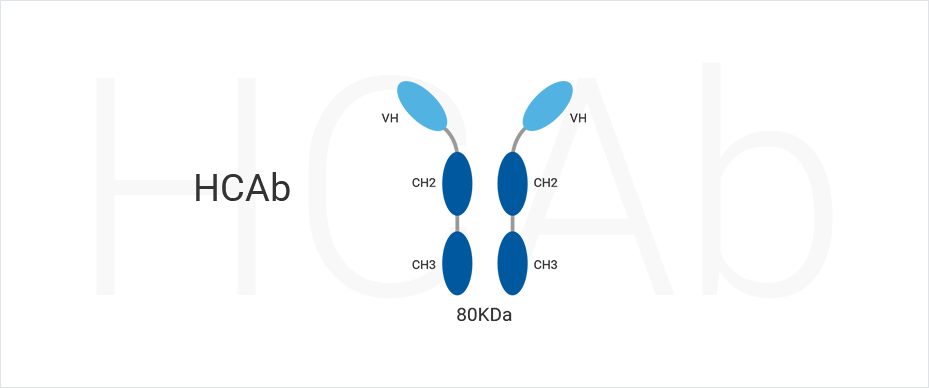
HCAb's patented technology generates novel "heavy chain only" antibodies, which are about half the size of a typical IgG. These antibodies carry IgG-like PK properties and Fc-domain functions without the need for additional engineering or humanization. Lack of light chain also minimizes the problem of light chain mispairing and heterodimerization. These characteristics enable the development of products with attributes not achievable by conventional antibody platforms. In addition, various formats, including single-domain antibodies, bi- and multi-specifics, antibody-drug conjugates, CAR-Ts or VH domain-derived diagnostic or therapeutic products, are also achievable using this platform.
Both of these platforms have significant potential to generate potential therapeutic antibodies and accelerate drug discovery and development, which has been validated through more than 40 licenses to pharmaceutical and biotechnology companies as well as academic institutions.
-
Desirable Solubility and Thermal Stability.
-
Excellent Human VH Diversity.
The antibody formats produced by the HCAb mice have shown exceptional diversity from selected, frequently expressed and soluble human V-gene germline families. Immunized HCAb mice are able to yield non-identical, highly diverse collection of antibodies that recognize different epitopes, or binding regions, on the same target protein. Total variability of HCAb antibody panel can be increased simply by screening more antibodies from more immunized HCAb mice.
-
Superior Potency.
The HCAbs generated on our HCAb Platform have been observed to have a higher range of binding affinity (nanomolar to picomolar binders) which are significantly higher than those from competing technologies such as naive phage display libraries.
-
Rapid and Efficient Discovery.
Due to the single chain nature of the HCAbs, they can be rapidly and deeply mined following immunization without the use of hybridoma technology, making them ready to progress very quickly into drug development. In addition, our fully human antibodies do not require any humanization, a process that at times has proven to be challenging and time consuming, and can result in antibodies with lowered binding affinities for their respective targets.
-
Wide Potential Applications.
When derived from the HCAb mice, the HCAbs are easily manipulated into making VH domain only molecules, bi-specifics or multi-specifics, VH domain-derived diagnostic or therapeutic molecules.
The versatility of our HCAb Platform is best demonstrated by our creation of HBM4003, our HCAb-based next-generation anti-CTLA-4 monoclonal antibody, as a proof of concept story in IND enabling studies, and its short journey to the Phase 1 clinical trial in less than three years from the target identification.
Publications
1. Dekker S, Toussaint W, Panayotou G, de Wit T, Visser P, Grosveld F, et al. Intracellularly Expressed Single-Domain Antibody against p15 Matrix Protein Prevents the Production of Porcine Retroviruses. J Virol. 2003;77(22):12132–9. (full text)
2. Janssens R, Dekker S, Hendriks RW, Panayotou G, van Remoortere A, San JK, et al.Generation of heavy-chain-only antibodies in mice. Proc Natl Acad Sci U S A. 2006;103(41):15130–5.(full text)
3. Laventie B-J, Rademaker HJ, Saleh M, de Boer E, Janssens R, Bourcier T, et al. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc Natl Acad Sci U S A. 2011;108(39):16404–9. (full text)
4. Drabek D, Janssens R, de Boer E, et al. Expression Cloning and Production of Human Heavy-Chain-Only Antibodies from Murine Transgenic Plasma Cells. Front Immunol. 2016;7:619.(full text)

Harbour BioMed has established a robust pipeline in oncology and immunology since the company was established in 2016. The current portfolio includes five clinical-stage in-licensed compounds and a rapidly emerging set of therapeutics coming from its internal discovery efforts as well as co-discovery/development collaborations with academic institutions and biopharmaceutical companies.
contact us





